Chemical characteristics and evolutionary mechanism of hot spring water in Dabie Mountain area, Anhui Province
-
摘要:研究目的
分析总结受构造控制的安徽大别山区温泉的水化学特征及其演化机制,有利于加深对独特地质背景下(造山带硅酸盐岩)水-岩相互作用的认识,同时可以为大别山区地热的勘探和合理开发利用提供科学依据。
研究方法在对研究区温泉水化学组分基本特征分析的基础上,综合利用Gibbs图、岩石风化图、离子比例系数、矿物稳定场图等方法对研究区温泉的水-岩相互作用进行研究。此外,还借助PHREEQC软件开展反向水文地球化学模拟工作,对地热水在循环过程中主要矿物的溶解及沉淀情况进行定量分析。
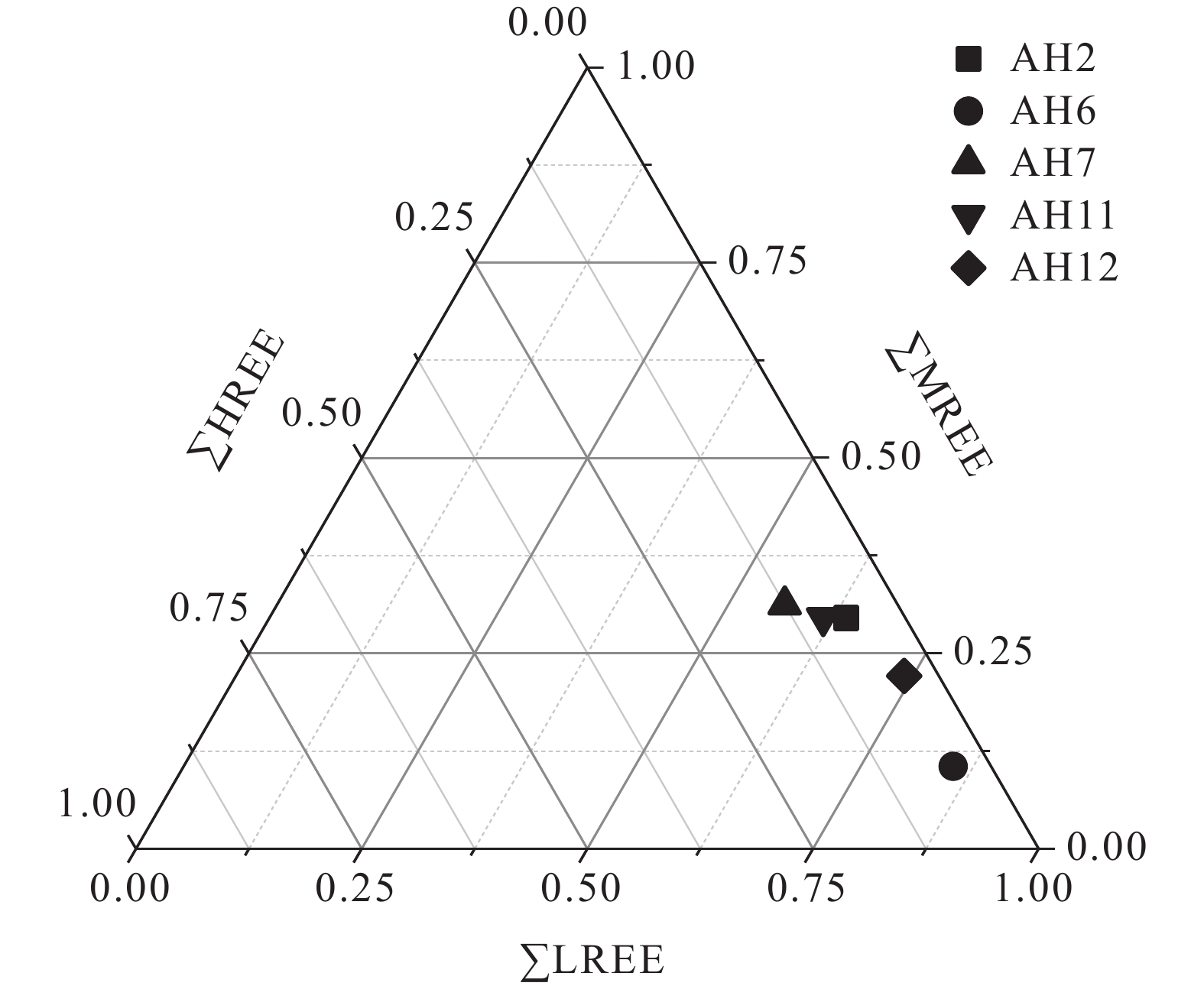
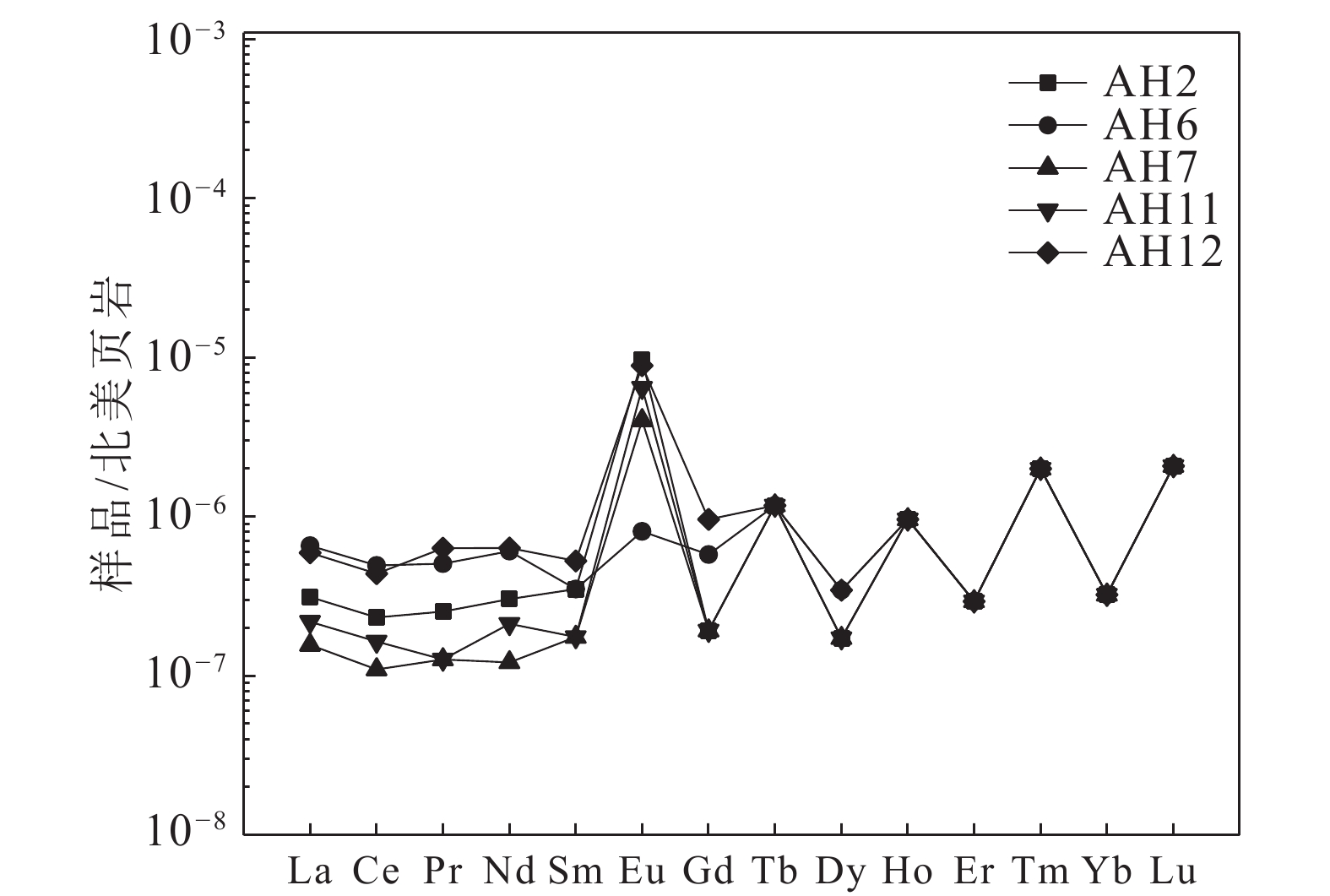
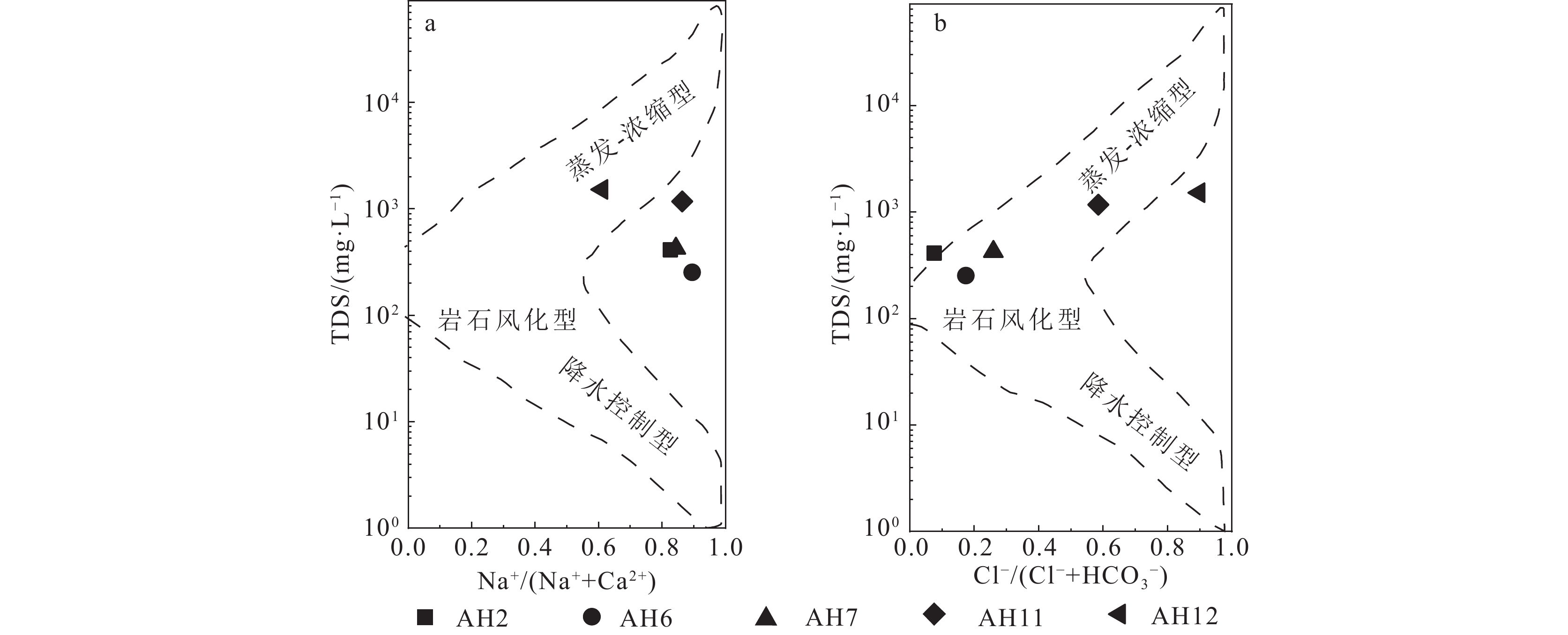
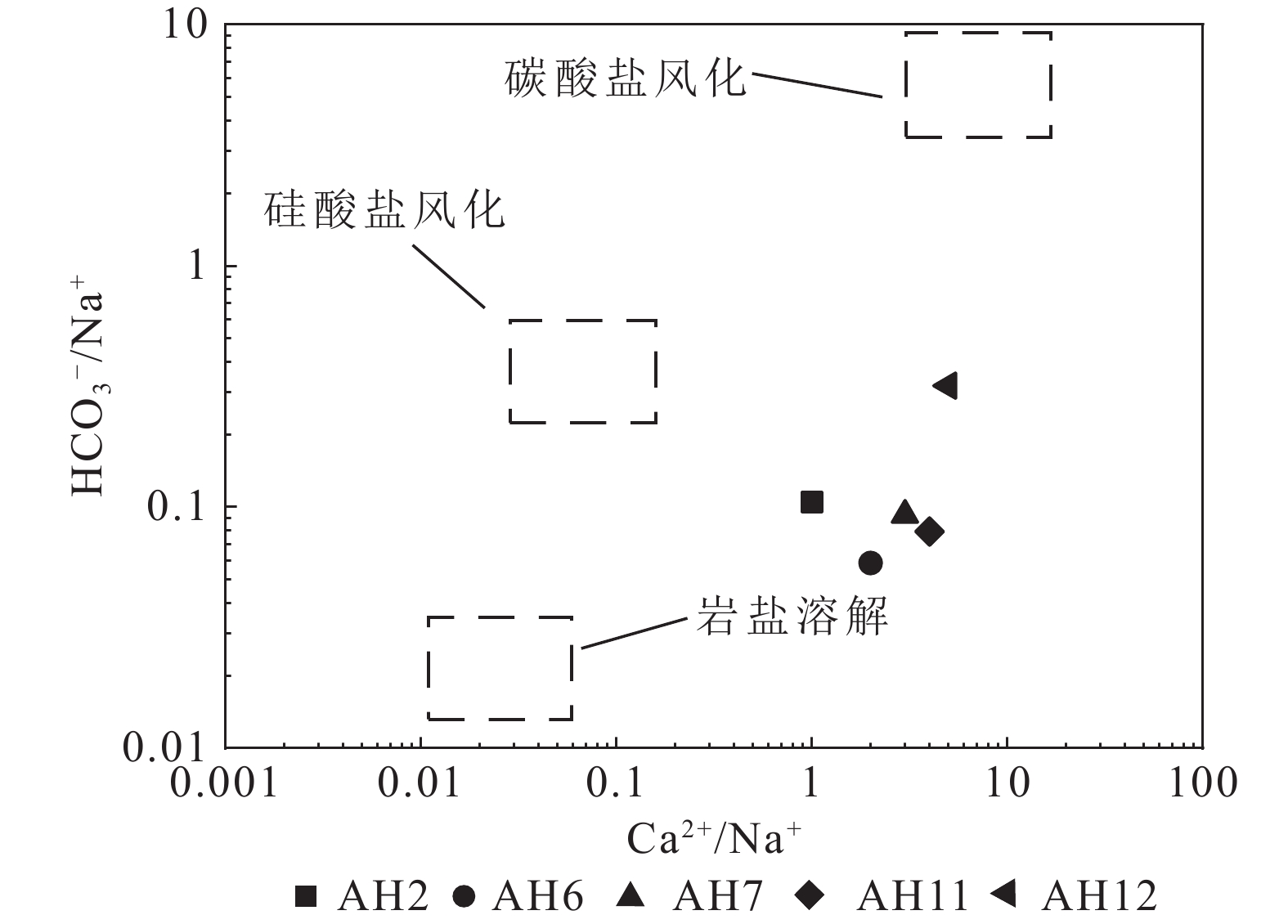
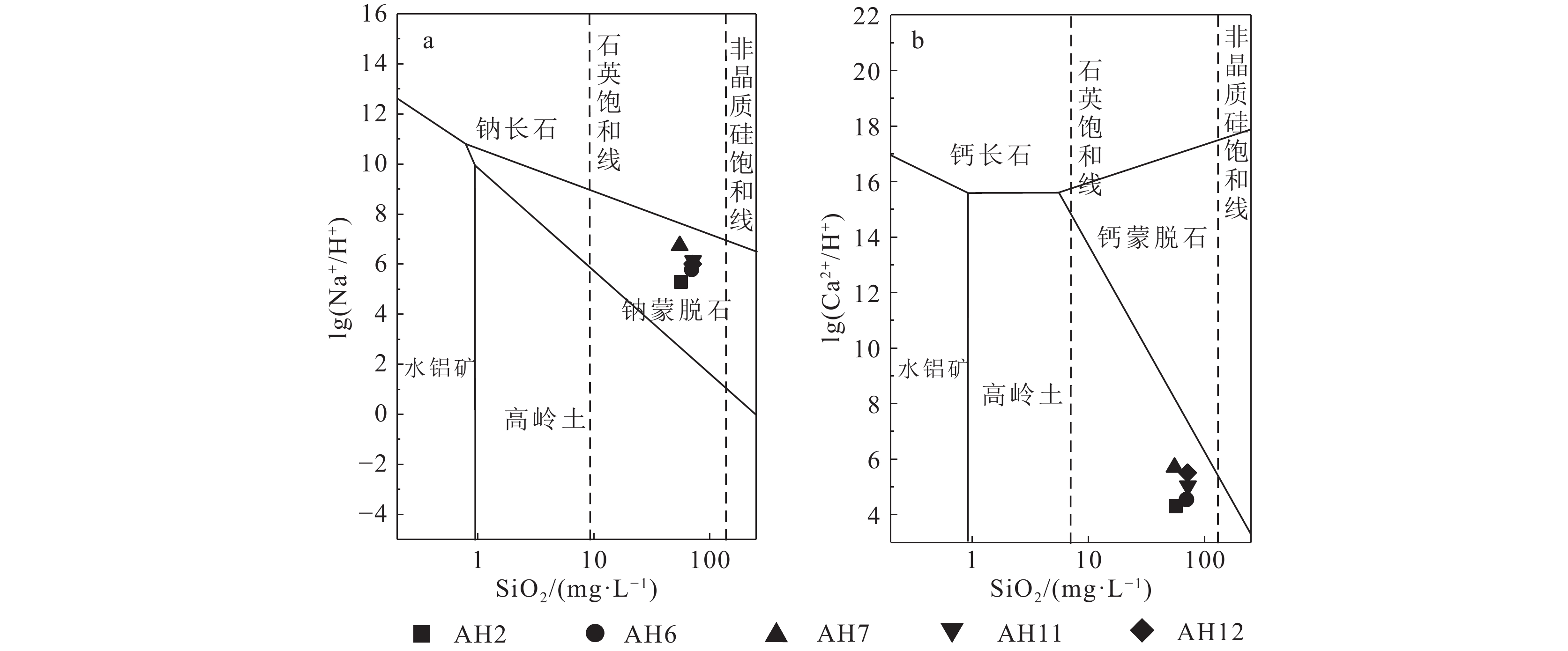
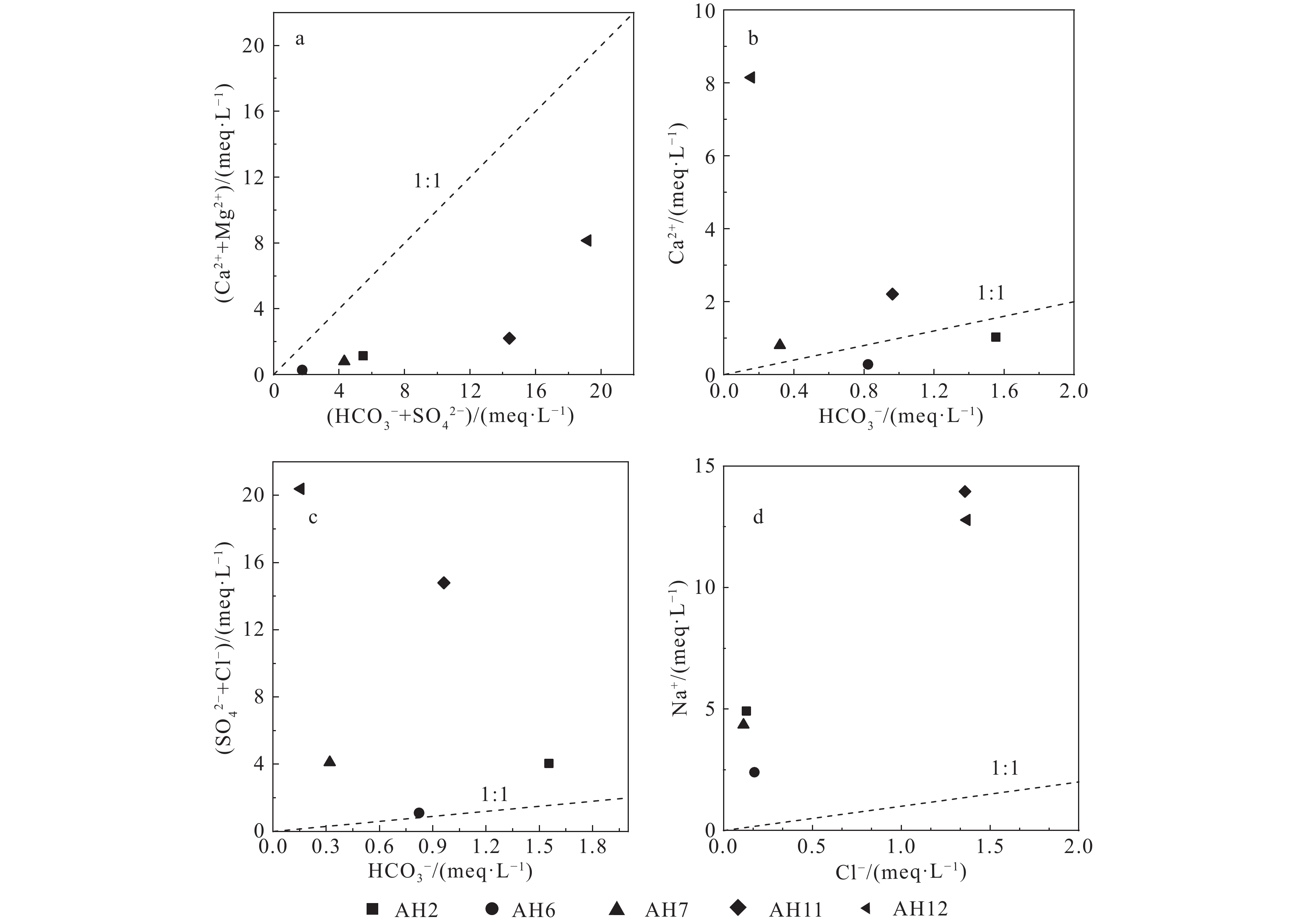
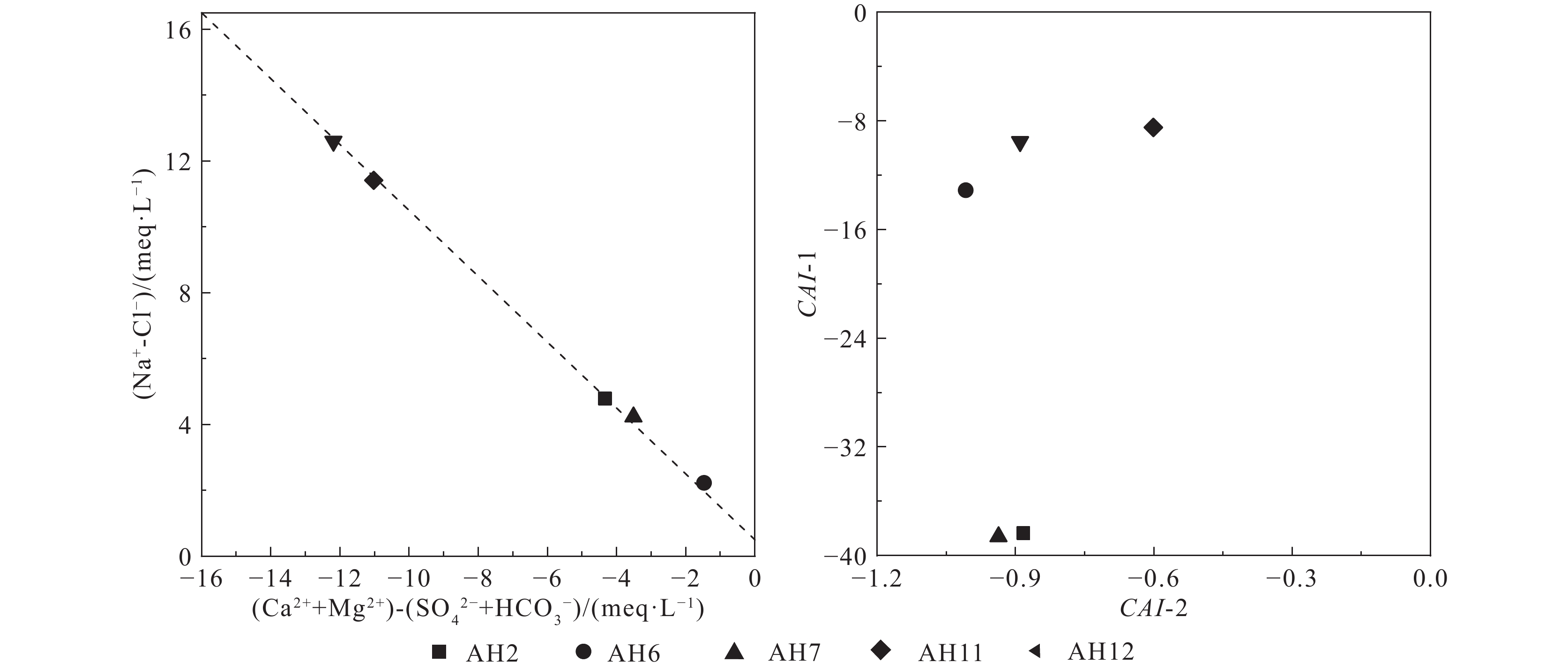
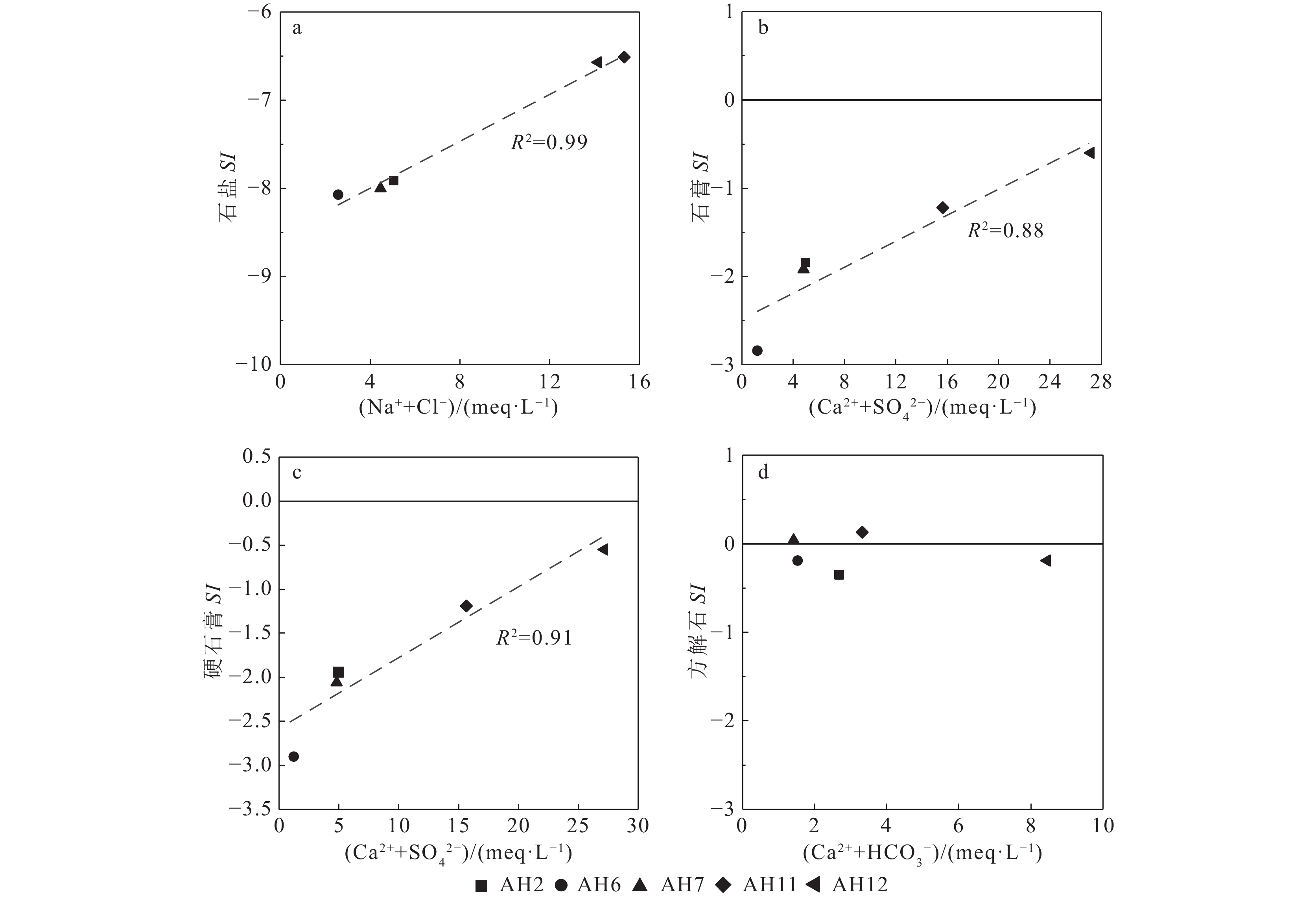
研究结果①大别山区5个温泉的水化学类型以SO4−Na型和SO4·HCO3−Na型水为主,均为中低温、弱碱性温泉;②研究区温泉中稀土元素表现出明显的Eu正异常,轻稀土元素相对富集,中稀土元素其次,重稀土元素相对缺乏;③研究区温泉水化学组分主要受岩石风化作用的影响。温泉中Na+主要来自硅酸盐矿物(如钠长石、钠蒙脱石等)的溶滤,Ca2+来源于碳酸盐矿物和石膏的溶滤;SO42−的含量主要受到石膏溶解的影响;HCO3−含量主要受硅酸盐和碳酸盐矿物溶解的影响。④模拟结果表明,雨水-地下热水路径上发生的水岩相互作用主要为钠长石、钙长石、萤石、石膏、黑云母和CO2的溶解,以及钠蒙脱石、方解石和白云石的沉淀,同时发生了Ca2+置换Na+的阳离子交替吸附作用。
结论雨水至地下热水路径属于地下水深循环,复杂的深部地层岩性及结构容易阻碍地下水径流,使地下水流速放缓,发生了充分的水-岩相互作用,完成了HCO3−Ca型雨水向SO4·HCO3−Na型和SO4−Na型弱碱性温泉的转化。
Abstract:ObjectiveThe analysis and summary of the hydrochemical characteristics and evolution mechanism of the tectonically controlled hot springs in Dabie Mountain area, Anhui Province, is conducive to deepen the understanding of water−rock interaction under the unique geological background (orogenic silicic rocks), and can provide scientific basis for geothermal exploration and rational development and utilization in Dabie Mountain area.
MethodsBased on the analysis of the basic characteristics of the chemical components of the hot spring in the study area, the water−rock interaction of the hot spring in the study area is studied by comprehensive use of Gibbs map, rock weathering map, ion ratio coefficient and mineral stability field map. In addition, the reverse hydrogeochemical simulation work was carried out with the help of PHREEQC software to quantitatively analyze the dissolution and precipitation of major minerals during the geothermal water cycle.
Results① The hydrochemical types of the five hot springs in Dabie Mountain are mainly SO4−Na and SO4·HCO3−Na, all of which are moderate−low temperature and weak alkaline hot springs; ② Eu values in hot springs in the study area show obvious positive anomalies, with light rare earth elements relatively abundant, medium rare earth elements second, and heavy rare earth elements relatively lacking; ③ The chemical composition of hot spring water in the study area is mainly affected by rock weathering. Na+ mainly comes from the leaching of silicate rocks (such as albite and sodium montmorillonite), and Ca2+ comes from the leaching of carbonate rocks and gypsum. The content of SO42− is mainly affected by the dissolution of gypsum. The content of HCO3− is mainly affected by the dissolution of silicate rocks and carbonate rocks. ④ The water−rock interaction on the path of rainwater−deep circulation underground hot water is the dissolution of albite, anorthite, fluorite, gypsum, biotite , and CO2, and the precipitation of sodium montmorillonite, calcite , and dolomite, and the cation alternating adsorption of Ca2+ replacing Na+ occurs.
ConclusionsThe path from rainwater to underground hot water belongs to the groundwater depth cycle. The complex lithology and structure of deep strata easily hinder groundwater runoff, slowing down groundwater velocity, and thus promote sufficient water−rock interaction in groundwater, completing the transformation of HCO3−Ca rainwater into SO4·HCO3−Na and SO4−Na weakly alkaline hot springs.
创新点(1)综合利用Gibbs图、岩石风化图、硅酸平衡图和离子比例系数等图解法,分析温泉深循环过程中发生的水-岩相互作用和主要的物质来源。(2)利用PHREEQC软件进行水化学模拟,对研究区经深循环形成的温泉的水-岩相互作用及水化学组分的迁移和富集进行定量分析。
-
-
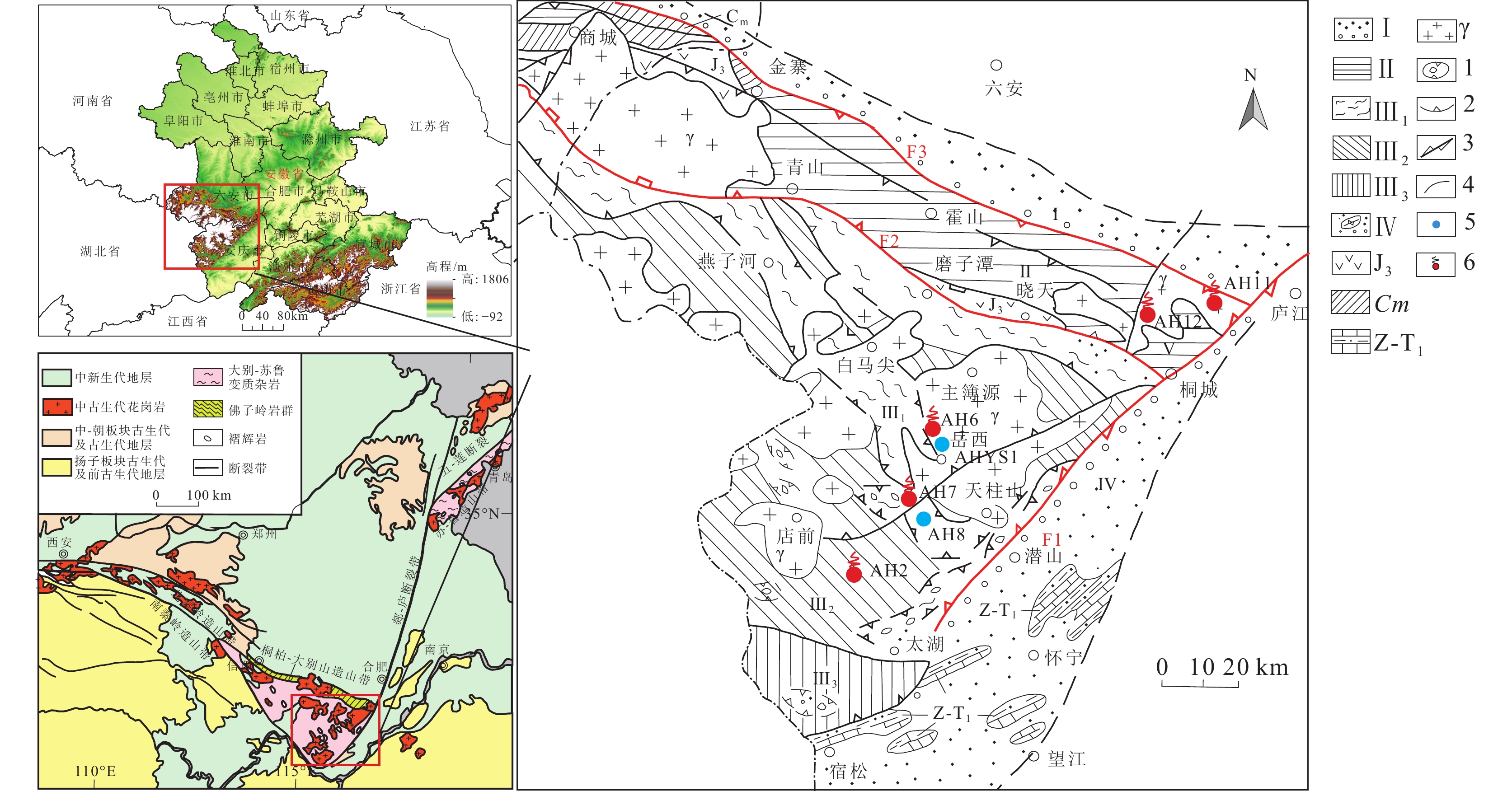
图 1 安徽大别山区地质略图(据徐树桐等,1992修改)
Ⅰ—江淮台隆和后继盆地;Ⅱ—北淮阳地槽褶皱带;Ⅲ—大别山中深变质杂岩带;Ⅲ1—罗田-岳西变质杂岩带;Ⅲ2—英山-潜山超高压变质岩带;Ⅲ3—宿松变质岩带和张八岭构造滑脱带;Ⅳ—前陆褶皱冲断带和磨拉斯盆地;Z-T1—卷入前陆褶皱冲断带的震旦系−下三叠统;Cm—石炭系梅山群;J3—晚侏罗世安山岩;γ—花岗岩;1—榴辉岩类;2—冲断带;3—走滑断层;4—地质界线;5—雨水样点及编号;6—温泉采样点及编号
Figure 1. Simplified geologic map of the Dabie Mountain area in Anhui
图 11 安徽大别山区温泉成因模式示意剖面图(据李状等,2022)
1—花岗岩(表层风化);2—大气降水入渗补给;3—示意性地下水流向;4—温泉;5—大地热流;6—破碎带
Figure 11. Schematic profile showing the formation of the hot springs in the Dabie Mountain area, Anhui Province
表 1 安徽大别山区温泉水化学组分
Table 1 Chemical composition of hot spring water in Dabie Mountain, Anhui Province
化学组分 单位 AH2 AH6 AH7 AH11 AH12 AHYS1 K+ mg/L 5 1.72 3.32 13.7 7.75 2.41 Na+ mg/L 113 55.2 100 321 294 0.96 Ca2+ mg/L 20.6 5.63 16.1 44.2 163 14.6 Mg2+ mg/L 1.31 0.03 0.03 0.03 0.03 0.37 Fe mg/L 0.19 0.06 0.03 0.05 0.06 0.05 HCO3− mg/L 94.8 50.2 19.5 58.7 9.48 43.4 CO32− mg/L 2.72 12.6 8.71 4.32 4.17 0 Cl− mg/L 4.61 6.24 4.03 48.9 49.3 0.76 SO42− mg/L 188 44.6 192 645 913 4.47 F− mg/L 5.56 3.38 6.5 8.02 3.86 0.1 NO3− mg/L 0.04 4.96 0.04 0.04 0.04 0.04 TDS mg/L 411 253 426 1172 1518 79 偏硅酸 mg/L 72.73 90.26 71.26 93.02 92.16 8.45 游离CO2 mg/L 0.66 0.09 总硬度 mg/L 56.83 14.06 40.2 110 407 37.96 Li μg/L 104 20.9 69.7 255 181 0.73 Sr μg/L 706 118 543 2238 5659 168 Zn μg/L 14.9 31.4 3.69 12.5 39.1 34.1 Ba μg/L 37.2 2.99 22.9 33.8 37.7 37.8 Cr μg/L 3.76 1.87 1.22 2.6 1.05 2.03 Mn μg/L 1.17 0.69 0.18 14.8 9.26 13.4 Ni μg/L 1.34 1.11 1.02 2.48 9.57 1.27 V μg/L 0.43 4.1 0.1 0.86 0.75 0.4 Eh mV −109 −80 −147 −152 −160 169 标高 m 80 408 120 40 100 408 T ℃ 46.3 51 40 61.3 64 30.6 pH 7.8 8.8 8.8 8 7.9 7.8 水化学类型 SO4·HCO3−Na SO4·HCO3−Na SO4−Na SO4−Na SO4−Na HCO3−Ca 表 2 矿物的溶解反应方程式
Table 2 Dissolution reaction equation of minerals
序号 矿物 反应式 1 钠长石 7NaAlSi3O8+6H++20H2O=3Na0.33Al2.33Si3.67O10(OH)2(钠蒙脱石)+6Na++10Si(OH)4 2 钙长石 CaAl2Si2O8+2H2CO3+H2O=Ca2++2HCO3−+Al2Si2O5(OH)4(高岭石) 3 钠蒙脱石 3Na0.33Al2.33Si3.67O10(OH)2+30H2O+6OH−=Na++7Al(OH)4−+11H4SiO4 4 石膏 CaSO4·2H2O=Ca2++2SO42− 5 方解石 CaCO3+H+=Ca2++HCO3− 6 白云石 CaMg(CO3)2+2H+=Ca2++Mg2++2HCO3− 7 萤石 CaF2=Ca2++2F− 8 黑云母 KMg3AlSi3O10(OH)2+6H++4H2O= K++3Mg2++Al(OH)4−+3H4SiO4 9 CO2 CO2(g)+H2O=H2CO3(aq);H2CO3=H++HCO3− 10 Na+/Ca2+ Ca2++2NaX=2Na++CaX2 2Na++CaX2=2NaX+Ca2+ 表 3 可能矿物相在各路径上的溶解-沉淀量
Table 3 Dissolution-precipitation amount of possible mineral phases in each path
mmol/L 矿物相 分子式 路径Ⅰ 路径Ⅱ 路径Ⅲ AHYS1-AH2 AHYS1-AH6 AHYS1-AH7 钠长石 NaAlSi3O8 0.894 1.726 2.871 钙长石 CaAl2Si2O8 0.5924 1.371 3.007 钠蒙脱石 3Na0.33Al2.33Si3.67O10(OH)2 −0.9206 −1.91 −3.824 方解石 CaCO3 −0.3552 −1.729 −3.836 白云石 CaMg(CO3)2 −0.16 0.0389 −0.09606 萤石 CaF2 0.1438 0.0864 0.1686 石膏 CaSO4:2H2O 1.95 0.4875 1.974 黑云母 K(Mg,Fe)3AlSi3O10(F,OH)2 0.0663 −0.01764 0.027 CO2(g) CO2 1.595 1.937 3.514 Na+/Ca2+ 4.028 0.9418 2.347 注:正值表示溶解或Ca置换Na进入水中,负值表示沉淀或Na置换Ca进入水中;CO2(g)表示CO2以气体相参与水化学反应 -
Anhui Geological Survey. 2006. 1∶250, 000 Regional Geological Survey of Taihu Formation, Anhui Province [M]. Beijing: Geological Press(in Chinese).
Auqué L F, Acero P, Gimeno M J, et al. 2009. Hydrogeochemical modeling of a thermal system and lessons learned for CO2 geologic storage[J]. Chemical Geology, 268(3/4): 324−336.
Bretzler A, Osenbruck K, Gloaguen R, et al. 2011. Groundwater origin and flow dynamics in active rift systems − A multi−isotope approach in the Main Ethiopian Rift[J]. Journal of Hydrology Amsterdam, 402(3/4): 274−289.
Cherdyntsev V V. 1971. Uranium−234[M]. Jerusalem: Keter Press.
Cheng C G, Yang L B. 2005. Preliminary Study on Distribution Characteristics and Development and Utilization of Geothermal Water Resources in Anqing City[J]. Geology of Anhui, 15(3): 186−189(in Chinese with English abstract).
China Geological Survey. 2012. Handbook of Hydrogeology[M]. Beijing: Geological Press(in Chinese).
Craig H. 1953. The geochemistry of the stable carbon isotopes[J]. Geochimica et Cosmochimica Acta, 3(2): 53−92.
Diao T R, Du F. 2019. Genesis model of geothermal resources and evaluation of geothermal resources in Yuexi County, Anhui Province[J]. Groundwater, 41(6): 27−28,31(in Chinese with English abstract).
Fisher R S, Mullican I W F. 1997. Hydrochemical evolution of sodium−sulfate and sodium−chloride groundwater beneath the northern Chihuahuan Desert, Trans−Pecos, Texas, USA[J]. Hydrogeology Journal, 5(2): 4−16. doi: 10.1007/s100400050102
Gaillardet J, Dupre B, Louva P, et al. 1999. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers[J]. Chemical Geology, 159(1/4): 3−30. doi: 10.1016/S0009-2541(99)00031-5
Gastmans D, Hutcheon I, Menegário A A, et al. 2016. Geochemical evolution of groundwater in a basaltic aquifer based on chemical and stable isotopic data: Case study from the Northeastern portion of Serra Geral Aquifer, São Paulo state (Brazil)(Article)[J]. Journal of Hydrology, 535(0): 598−611.
Gibbs R J. 1970. Mechanisms controlling world water chemistry[J]. Science, 170(3962): 1088−1090. doi: 10.1126/science.170.3962.1088
Guo Y H, Shen Z L, Zhong Z S. 2002. Hydrogeochemical modeling for the formation of deep−lying alkaline fresh groundwater in Heibei Plain: A case study in Baoding and Cangzhou Districts[J]. Earth Science, 27(2): 35−40(in Chinese with English abstract).
Haskin L A, Haskin M A, Frey F A, et al. 1968. Relative and Absolute Terrestrial Abundances of the Rare Earths[M]. London: Pergamon Press.
Helgeson H C. 1969. Evaluation of irreversible reactions in geochemical processes involving minerals and aqueous solutions-II. Applications[J]. Geochimica et Cosmochimica Acta, 32(8): 853-877.
Hershey R L, Mizell S A, Earman S. 2010. Chemical and physical characteristics of springs discharging from regional flow systems of the carbonate−rock province of the Great Basin, western United States[J]. Hydrogeology Journal, 18(4): 1007−1026. doi: 10.1007/s10040-009-0571-7
Hidalgo M C, Cruz−Sanjulian J. 2001. Groundwater composition, hydrochemical evolution and mass transfer in a regional detrital aquifer (Baza basin, southern Spain)[J]. Applied Geochemistry, 16(7): 745−758.
Li Z, Zhou X, Fan B, et al. 2022. Hydrochemical and isotopic characteristics and formation of the hot spring in the Dabie Mountain area, Anhui Province[J]. Geological Bulletin of China, 41(9): 1687−1697(in Chinese with English abstract).
Lin W J, Liu Z M, Wang W L, et al. 2013. The assessment of geothermal resources potential of China[J] Geology in China, 40(1): 312−321(in Chinese with English abstract).
Liu H Y. 2018. Distribution of groundwater rare earth elements in the typical region of the North China Plain and modeling study on their complexation with iron and manganese[D]. Doctoral Thesis of China University of Geoscience (Beijing) (in Chinese with English abstract).
Liu C L, Li Y S, Hong B Y, et al. 2023. Geochemical characteristics and formation mechanisms of the seawater−recharged geothermal systems in Yantian of Fujian, China[J]. Hydrogeology & Engineering Geology, 50(1): 158−167(in Chinese with English abstract).
Michard G, Roekens E. 1983. Modeling of the chemical composition of alkaline hot waters[J]. Geothermics, 12(12/13): 161−169.
Nesbitt H W, Young G M. 1984. Prediction of some weathering trends of plutonic and volcanic rocks based on thermodynamic and kinetic considerations[J]. Geochimica et Cosmochimica Acta, 48(7): 1523−1534. doi: 10.1016/0016-7037(84)90408-3
Palandri J L, Reed M. 2001. Reconstruction of in situ composition of sedimentary formation waters[J]. Geochimica et Cosmochimica Acta, 65(11): 1741−1767. doi: 10.1016/S0016-7037(01)00555-5
Parkhurst D, Appelo C. 1999. User's guide to PHREEQC (Version 2)−A Computer Program for Speciation, Batch−reaction, One−dimensional Transport and Geochemical Calculations[M]. U. S: Geological Survey Water Resour Invest Rep, 99−4259.
Qi H, Ma Z Y, Li Pei Y, et al. 2012. Hydrogeochemical[M]. Beijing: Geology Press(in Chinese).
Rajmohan N, Elango L. 2004. Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India[J]. Environmental Geology, 46(1): 47−61.
Reed M, Spycher N. 1984. Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution[J]. Geochimica et Cosmochimica Acta, 48(7): 1479−1492. doi: 10.1016/0016-7037(84)90404-6
Rosenthal E, Jones B F, Weinberger G. 1998. The chemical evolution of Kurnub Group paleowater in the Sinai−Negev province—A mass balance approach[J]. Applied Geochemistry, 13(5): 553−569. doi: 10.1016/S0883-2927(97)00092-9
Reddy A G S, Kumar K N. 2010. Identification of the hydrogeochemical processes in groundwater using major ion chemistry: A case study of Penna−Chitravathi river basins in Southern India[J]. Environmental Monitoring and Assessment, 170(1/4): 365−382.
Rogers R J. 2010. Geochemical comparison of ground water in areas of New England, New York, and Pennsylvania[J]. Ground Water, 27(5): 690−712.
Sakai T, Yamaguchi A, Metz P. 2003. Thermal−hydraulic analysis for a sodium−heated steam generator using a multi−shell method[J]. Nuclear Engineering and Design, 219(1): 35−46. doi: 10.1016/S0029-5493(02)00212-1
Sanliyuksel D, Baba R. 2011. Hydrogeochemical and isotopic composition of a low−temperature geothermal source in northwest Turkey: Case study of Kirkgecit geothermal area[J]. Environmental Earth Sciences, 62(3): 529−540. doi: 10.1007/s12665-010-0545-z
Schoeller H. 1965. Hydrodynamique lans lekarst (ecoulemented emmagusinement)[J]. Actes Colloques Doubronik, 1(0): 3−20.
Stallard R F, Edmond J M. 1983. Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load[J]. Journal of Geophysical Research: Oceans, 88(14): 9671−9688.
Tardy Y. 1971. Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs[J]. Chemical Geology, 7(4): 253−271. doi: 10.1016/0009-2541(71)90011-8
Ta M M, Zhou X, Guo J, et al. 2020. The Evolution and Sources of Major Ions in Hot Springs in the Triassic Carbonates of Chongqing, China[J]. Water, 12(4): 1194. doi: 10.3390/w12041194
Tang Y X, Lin J W, Li Y Y, et al. 2024. The occurrence regularity of deep geothermal resources in the northern of Binhai geothermal field, Tianjin[J]. North China Geology, 47(1): 77−84(in Chinese with English abstract).
Uliana M M, Sharp J M. 2001. Tracing regional flow paths to major springs in Trans−Pecos Texas using geochemical data and geochemical models[J]. Chemical Geology, 179(1): 53−72.
Wang Y P, Wang L, Xu C X, et al. 2010. Hydro−geochemistry and genesis of major ions in the Yangtze River, China[J]. Geological Bulletin of China, 29(2/3): 446−456(in Chinese with English abstract).
Wang M M. 2020. Hydrochemical and isotopic characteristics of geothermal water in the Rehai region in Tengchong of Yunnan[D]. Doctoral Thesis of China University of Geoscience (Beijing) (in Chinese with English abstract).
Wang X C, Sun H L, Yuan X F. 2022. A study of the hydrochemical characteristics and geothermal water of typical granite geothermal reservoir in the Jiaodong area[J]. Hydrogeology & Engineering Geology, 49(5): 186−194(in Chinese with English abstract).
Wang X J, Xu M J, Han X, et al. 2023. Study on Groundwater Cycle in Beijing Pinggu Basin Based on Isotopes and Hydrochemistry[J]. Northwest Geology, 56(5): 127−139(in Chinese with English abstract).
Wang B, Zong Z H, Xia Y B, et al. 2023. The characteristics of the main ion components of geothermal fluid and geothermal origin analysis in Tianjin[J]. North China Geology, 46(2): 9−16(in Chinese with English abstract).
Wu H Q, Yang Z D, Shu Q, et al. 2016. Distribution characteristics of geothermal resources in Anhui Province and their development and utilization suggestions[J]. Journal of Geology, 40(1): 171−177(in Chinese with English abstract).
Wu H Q, Li Q, Fan D W. 2019. Discussion of Petrological and Geochemical Characteristics and Formation of Xiangchang Super unit in Anhui Dabie Mountains[J]. Journal of Suzhou University, 34(9): 66−72(in Chinese with English abstract).
Wu J Y, Liu H, OuYang Y, et al. 2023. Hydrochemical Characteristics and Water Quality Assessment of Groundwater in Northern Foothill of Luoji Mountains[J]. Northwest Geology, 56(5): 151−164(in Chinese with English abstract).
Xi L, Chen K H, Huang X Q, et al. 2021. Hydrogeochemistry and origin of groundwater in the south coast of Hainan[J]. Geological Bulletin of China, 40(2/3): 350−363(in Chinese with English abstract).
Xu S T, Jiang L L. 1992. Tectonic framework and evolution of the Dabie Mountain in Anhui, eastern China[J]. Acta Geologica Sinica, 66(1): 1−14(in Chinese with English abstract).
Yang N, Wang G C, Shi Z, et al. 2018. Application of Multiple Approaches to Investigate the Hydrochemistry Evolution of Groundwater in an Arid Region: Nomhon, Northwestern China[J]. Water, 10(11): 1667. doi: 10.3390/w10111667
Zhang R Q, Liang X, Jin M G, et al. 2018. The Fundamentals of Hydrogeology[M]. Beijing: Geological Publishing House (in Chinese).
Zhou Z Y, Liu S L, Liu J X. 2015. Study on the Characteristics and Development Strategies of Geothermal Resources in China[J]. Journal of Natural Resource, 30(7): 1210−1221(in Chinese with English abstract).
Zhou X, Jin X M, Liang S H, et al. 2017. Special Topics on Groundwater Sciences (the second editon) [M]. Beijing: Geological Publishing House(in Chinese).
Zhu X H, He Tao, Wu Q. 2020. Analyzing the causes of Tangwan Hot Spring in Taihu County, Anhui Province[J]. Western Resource, (4): 132−134(in Chinese).
安徽省地质调查院. 2006. 安徽省太湖幅1∶25万区域地质调查[M]. 北京: 地质出版社. 程长根, 杨立本. 2005. 安庆市地热水资源分布特征与开发利用初步研究[J]. 安徽地质, 15(3): 186−189. doi: 10.3969/j.issn.1005-6157.2005.03.005 刁天仁, 杜菲. 2019. 安徽省岳西县溪沸地热成因模式及地热资源评价[J]. 地下水, 41(6): 27−28,31. 郭永海, 沈照理, 钟佐燊. 2002. 河北平原深层碱性淡水形成的水文地球化学模拟——以保定、沧州地区为例[J]. 地球科学, 27(2): 35−40. 李状, 周训, 方斌, 等. 2022. 安徽大别山区温泉的水化学与同位素特征及成因[J]. 地质通报, 41(9): 1687−1697. doi: 10.12097/j.issn.1671-2552.2022.09.016 蔺文静, 刘志明, 王婉丽, 等. 2013. 中国地热资源及其潜力评估[J]. 中国地质, 40(1): 312−321. doi: 10.3969/j.issn.1000-3657.2013.01.021 刘海燕. 2018. 华北平原典型区地下水稀土元素的分布特征及其与铁、锰络合反应的模拟研究[D]. 中国地质大学(北京)博士学位论文. 刘春雷, 李亚松, 洪炳义, 等. 2023. 福建盐田海水补给型地热系统地球化学特征及其成因[J]. 水文地质工程地质, 50(1): 158−167. 钱会, 马致远, 李培月, 等. 2012. 水文地球化学[M]. 北京: 地质出版社. 唐永香, 林建旺, 李嫄嫄, 等. 2024. 天津滨海地热田北部深部地热资源赋存规律[J]. 华北地质, 47(1): 77−84. 王亚平, 王岚, 许春雪, 等. 2010. 长江水系水文地球化学特征及主要离子的化学成因[J]. 地质通报, 29(2/3): 446−456. doi: 10.3969/j.issn.1671-2552.2010.02.032 王蒙蒙. 2020. 云南腾冲市热海地区地下热水的水化学和同位素特征研究[D]. 中国地质大学(北京)博士学位论文. 王晓翠, 孙海龙, 袁星芳. 2022. 胶东典型花岗岩热储地下热水水化学特征及热储研究[J]. 水文地质工程地质, 49(5): 186−194. 王冰, 宗振海, 夏雨波, 等. 2023. 天津地区地热流体主要离子组分特征及地热成因分析[J]. 华北地质, 46(2): 9−16. 王新娟, 许苗娟, 韩旭, 等. 2023. 基于同位素和水化学的北京平谷盆地地下水循环研究[J]. 西北地质, 56(5): 127−139. doi: 10.12401/j.nwg.2022037 吴海权, 杨则东, 疏浅, 等. 2016. 安徽省地热资源分布特征及开发利用建议[J]. 地质学刊, 40(1): 171−177. doi: 10.3969/j.issn.1674-3636.2016.01.171 吴海权, 李琴, 范董伟. 2019. 安徽大别山响肠超单元的岩石学和地球化学特征及成因探讨[J]. 宿州学院学报, 34(9): 66−72. 吴君毅, 刘洪, 欧阳渊, 等. 2023. 螺髻山北麓地下水化学特征与水质评价[J]. 西北地质, 56(5): 151−164. doi: 10.12401/j.nwg.2023003 习龙, 陈科衡, 黄向青, 等. 2021. 海南南部沿海地下水水文地球化学及成因[J]. 地质通报, 40(2/3): 350−363. 徐树桐, 江来利, 刘贻灿, 等. 1992. 大别山区(安徽部分)的构造格局和演化过程[J]. 地质学报, 66(1): 1−14. 张人权, 梁杏, 靳孟贵, 等. 2018. 水文地质学基础(第七版)[M]. 北京: 地质出版社. 中国地质调查局. 2012. 水文地质手册[M]. 北京: 地质出版社. 中华人民共和国卫生部. 2006. 生活饮用水卫生标准(GB 5749-2006)[S]. 北京: 中国标准出版社. 中华人民共和国国家质量监督检验检疫总局, 国家标准化管理委员会. 2016. 天然矿泉水资源地质勘查规范(GB/T 13727—2016)[S]. 北京: 中国标准出版社. 中华人民共和国卫生部, 国家标准化管理委员会. 2018. 食品安全国家标准 饮用天然矿泉水标准(GB 8537—2018)[S]. 北京: 中国标准出版社. 周训, 金晓媚, 梁四海, 等. 2017. 地下水科学专论(第二版)[M]. 北京: 地质出版社. 周总瑛, 刘世良, 刘金侠. 2015. 中国地热资源特点与发展对策[J]. 自然资源学报, 30(7): 1210−1221. 朱训和, 何涛, 吴琼. 2020. 解析安徽省太湖县汤湾温泉成因[J]. 西部资源, (4): 132−134.



 下载:
下载: